Malaria Diagnostic Kit – an important tool to prevent deaths from malaria in Africa
Malaria is one of the diseases leading high numbers of morbidity and mortality in many countries, especially in Sub Saharan Africa. Malaria remains the cause of high morbidity and mortality rates in many countries. It alone covers 30% of outpatient admissions and accounts up to 5% of inpatient deaths while 170 million working days are lost annually because of it.
Kenya in 2004, bought a change in opting artemisinin-based combination therapy (ACT) as first-line treatment for malaria which can be uncomplicated. This led to more effective treatment and also led to the improvement of malaria case management which requires correct use of medicines and safeguarding that patients are correctly diagnosed and appropriately managed.
On October 4, 2012, malaria diagnostic test kits (MDT) nationally came into existence which was another landmark in the treatment of malaria. The launch was expected not only to increase the diagnosis of malaria using MDTs in the nation but also stimulate the proper use of anti-malarial medicines.
The management of malaria has previously been based on the clinical symptoms of a patient under five in malaria endemic zones. It became necessary to embrace a universal diagnostic policy for all age groups and all zones regardless of the endemicity to successfully provide universal malaria treatment.
Based on universal approvals, the Kenyan government accepted a policy of entire diagnosis of malaria for all ages and across all areas of the nation. Suspected malaria cases would be tested and those found to be on a positive end would be treated with the recommended anti-malarial therapy.
In an offer to reinforce malaria diagnosis and apply the policy, the ministries of health with funding from various development partners obtained approximately eight million RDTs for distribution and use in 2012 and a further eleven million is targeted for 2013.
The placement of diagnostics has in the past been earmarked for hospitals and large health facilities with microscopy services and with formally trained laboratory technicians and technologists. However, obtained RDTs kits will be circulated to all public healthcare services across the country with urgency given to facilities which presently do not provide other diagnostic services for malaria. The placement of RDTs by DOMC will expand diagnostic coverage all the way down to the pharmacies with a goal to roll them out to the community health workers in 2013.
HCSM is presently involved in training 3,200 frontline health workers on the use of RDTs in lower-level facilities nationwide as well as facilitates technical assistance to ensure that RDTs and other malaria supplies are managed correctly at the facility level.
Benefits of malaria diagnostic test kit
The RDTs signify profits through all areas of the healthcare delivery system; specifically, patients are diagnosed and treated correctly within healthcare centers. With better services, predictors can better approximate the therapy needed and which also allows in saving extra costs on other medications.
For example a retail cost for Artemisinin/Lumefantrine (AL) 24s – used for malaria treatment – costs approximately $5 USD and a malaria rapid test kit costs less than $1 USD In this case a precise analysis saves the government or the patient $4 USD in unnecessary costs in treating patients with negative results.
Additionally, the danger of parasites becoming resistant to medicines is reduced if diagnosis-based treatment is been enforced. This eventually helps in raising the useful beneficial life of the available medicines and thus help in reducing the chances to follow other expensive alternative treatments.
When to use malaria diagnostic test kit
Malaria is caused by Plasmodium parasites which is known as mosquito-borne infectious disease. Malaria diagnostic test kit should be carried out for the detection of Malaria. If the result is positive, medical help must be immediately sought for a definitive clinical diagnosis and prompt treatment.
What is the malaria diagnostic test kit?
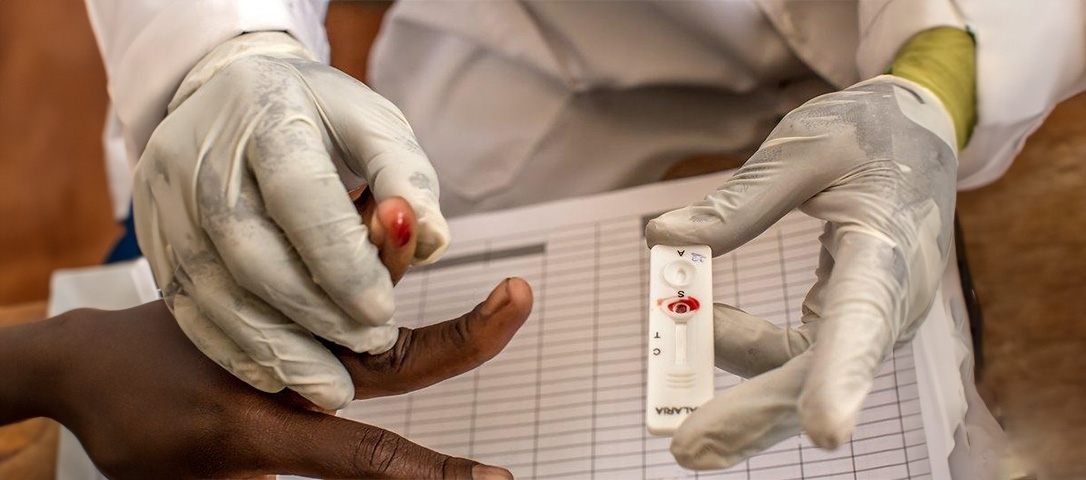
Malaria diagnostic test kit is a simple-to-use, fast, painless, reliable and accurate blood test. AdvaCare is a CE, ISO and USFDA manufacturer of Malaria Test Kits.
AccuQuik malaria test kits assist in the diagnosis of malaria by detecting evidence of malaria parasites (antigens) in human blood through the use of species-specific complementary markers in the test device.
The malaria test kits are available to detect both Plasmodium falciparum and Plasmodium vivax infection. It is a one-step test that takes approximately 20 minutes to provide a result. An individual with a positive result should consult a doctor for the first-line treatment.
Our malaria diagnostic test kits are accurate in 98% of cases, clearly demonstrating their reliability. Certain medical factors may influence the result of the test, for instance, an individual taking other medications should consult a doctor before taking the test.
Don't want to miss the next AdvaCare article?

Recommended Content

Pet Supplements Manufacturer: Functional and Premium Ingredients with USA Quality Standards & Traceability


Global Pet Supplement Market Trends: Why Distributors are Shifting to Premium Grade Quality


TickZero™: High-Margin, 12-Week Flea & Tick Protection, Distributor Opportunity in LMICs
