Spinal Needles
Type
Needle Size (color-coded)
Packaging
What are Spinal Needles?
Spinal Needles are used to access the subarachnoid space in the spinal cord. They are typically used for spinal anesthesia, to inject medication, or obtain cerebrospinal fluid samples for diagnosis. These needles are designed with a sharp bevel tip and a thin, long body to minimize tissue damage and maximize accuracy.
Precision needles are available in a variety of sizes and shapes to accommodate different procedures and patient needs. They are typically color-coded to indicate their gauge size, with larger gauge sizes denoted by lower numbers.
AdvaCare Pharma manufactures Spinal Needles in our facilities located in the USA, India, and China. Our production plants undergo regular inspection to ensure compliance with the highest standards for safety, health, and the environment, as well as ISO and CE regulations. We are committed to manufacturing high-quality precision needles for the global medical community.
Product Specifications
Type
Pencil Point
Pencil point spinal needles, also known as atraumatic needles, are specially designed to minimize the leakage of cerebrospinal fluid after puncture, thus reducing the likelihood of postdural puncture headache. They are characterized by their rounded tip, which reduces the risk of tissue trauma during insertion. These needles are commonly used for spinal anesthesia and diagnostic purposes.
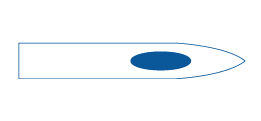
Quincke
Quincke spinal needle, also known as a conventional needle, is designed with a sharp tip to cut through tissue and a distal opening. It is commonly used for lumbar punctures to obtain cerebrospinal fluid samples or to administer spinal anesthesia. The beveled tip of the needle can cause some damage to the tissue, but it provides a quicker insertion into the dura mater compared to pencil point needles.
Why are we a trusted Spinal Needles manufacturer?
AdvaCare Pharma's Spinal Needles are designed with safety, patient comfort, and ease of use in mind. Our AccuPoint™ brand products, such as our spinal needles are manufactured in ISO and CE-certified facilities. Our rigorous quality control measures ensure that our partners receive only quality-assured medical devices.
We supply a wide range of medical devices to distributors, hospitals, pharmacies, NGOs, and government institutions in over 65 markets worldwide, and are committed to working with our partners to ensure that their needs are met.
As quality-focused spinal needle manufacturer, we understand the importance of timely delivery and strive to ensure that our products reach our medical distributors as quickly as possible through our global distribution network. Our dedicated teams of sales, regulatory and product specialists are available to provide support and answer any questions our partners may have.
Uses
How do Spinal Needles function in medical practice?
Spinal Needles assist in navigating to the subarachnoid space of the spinal cord, a major area for administering spinal anesthesia or extracting cerebrospinal fluid for diagnostic purposes:
- The procedure begins with an aseptic preparation of the patient and the use of precision-engineered needles for minimal discomfort and risk.
- The practitioner must choose the appropriate needle type—either a pencil point or Quincke—based on the specific needs of the procedure and patient anatomy.
- The needle's insertion follows a meticulous path to avoid contact with sensitive nerve fibers, aiming for the ideal entry point that allows access to the spinal canal while maintaining the integrity of surrounding tissues.
What distinguishes Pencil Point Needles from Quincke Needles?
Pencil Point Needles are designed with a non-cutting tip to part the dura mater fibers gently, aiming to mitigate the risk of post-dural puncture headaches—a common complication associated with spinal needle insertion. This design preference is beneficial for patient comfort and reducing the incidence of cerebrospinal fluid leaks.
On the other hand, Quincke Needles, characterized by their cutting bevel, offer a precise entry but may elevate the likelihood of tissue trauma and subsequent leakage. The choice between these needle types is influenced by the procedure and the practitioner’s judgment on minimizing patient discomfort while achieving the procedural goals.
How are sizes and color codes determined for Spinal Needles?
The sizing of Spinal Needles is all-important for matching the procedure's requirements to the patient's anatomical considerations. Needle gauge, an indicator of the needle's outer diameter, inversely correlates with the needle's size—the higher the gauge, the thinner the needle.
This precise differentiation allows medical professionals to select needles that provide the right balance between ease of insertion and patient comfort. The color-coding system, a standardized method of identification, enables quick recognition of needle sizes, facilitating efficient preparation and reducing the time to procedure.
What are the best practices for the maintenance and management of Spinal Needles?
Checking the sterility and integrity of Spinal Needles is paramount for patient safety and the success of spinal procedures. These needles should be stored in a controlled environment, safeguarded from potential contaminants and temperature extremes that could compromise their sterility.
Handling should prioritize aseptic technique, from the moment of unpackaging to the procedure's execution, to prevent infection risks. Post-use, the immediate and careful disposal of these needles into sharps containers is mandatory, not only to uphold safety protocols but also to respect environmental health standards.
Navigating the procedural application of Spinal Needles for diverse medical needs. Spinal Needles serve a broad spectrum of medical applications, from diagnostic lumbar punctures to the administration of anesthesia for surgeries that need profound numbness. The procedural application begins with an assessment of the patient's condition and the specific objectives of the spinal procedure. Factors such as the needle's gauge and length are tailored to the patient's body type and the procedural depth requirement.
Following the insertion, the practitioner's expertise in maneuvering the needle to the precise location insures the procedure's effectiveness while minimizing potential discomfort and complications.
How do medical professionals preserve the sterility of Spinal Needles?
Once in the clinical setting, healthcare providers must uphold the integrity of the sterile packaging until the moment of use, employing aseptic techniques throughout the procedure to prevent introduction of pathogens.
What training do practitioners receive for Spinal Needle procedures?
Practitioners undergo extensive training that encompasses both the theoretical knowledge of spinal anatomy and hands-on procedural techniques.
This education typically includes simulated practice on models to develop skill in needle insertion, cerebrospinal fluid withdrawal, and medication administration.
Advanced training also covers the management of potential complications and patient care post-procedure, assuring comprehensive competency in spinal needle use.
Can Spinal Needles be used for multiple types of spinal injections?
Yes, Spinal Needles are versatile tools designed for a variety of spinal injections, including anesthesia for surgeries, diagnostic lumbar punctures, and therapeutic injections for pain management.
The specific needle selection—taking into account the gauge, length, and tip type—is tailored to the nature of the injection, the viscosity of the substance being administered, and the depth of the targeted spinal area, allowing for flexibility across different spinal procedures.
What advancements have been made in Spinal Needle technology?
Recent advancements in Spinal Needle technology focus on enhancing patient safety and procedural efficacy. Innovations include the development of finer gauge needles that reduce tissue trauma and the integration of ergonomic features that elevate the control and precision of needle insertion.
There's been progress in materials engineering, creating needles that sustain sharpness and rigidity while minimizing patient discomfort during penetration and withdrawal.
FAQs
How do Spinal Needles work?
Spinal needles are used to access the subarachnoid space in the spinal cord to inject medication or obtain cerebrospinal fluid samples. The needle is inserted through the skin and into the spinal canal, and the medication is injected or the sample is collected.
What is the difference between pencil point and quincke Spinal Needles?
Pencil point needles are designed to create a small hole in the dura, while quincke needles have a bevel that cuts the dura to create a larger hole. Pencil point needles are less traumatic and associated with fewer complications, while quincke needles may be preferred in certain situations.
How are Spinal Needles sized and color-coded?
Spinal needles are sized by gauge, with larger numbers denoting a smaller diameter. They are also color-coded based on gauge size, with smaller gauges typically being green and larger gauges being pink or red.
How should Spinal Needles be stored and handled?
Spinal needles should be stored in a cool, dry place and kept in their original packaging until use. They should be handled with care to avoid bending or damaging the needle, and should be disposed of properly after use.
Are there any risks associated with Spinal Needle use?
Spinal needle use carries certain risks, such as nerve damage, bleeding, infection, and spinal headache. However, these risks can be minimized through proper technique and careful patient selection.
Is it possible to customize the specifications of your Spinal Needles according to my preferences?
We provide options to customize the configurations of numerous medical devices to meet unique user preferences and clinical demands. Leveraging our agile manufacturing techniques, we can enhance production capability to offer a broader spectrum of specifications, ensuring suitability for diverse market needs.
Do your Spinal Needles adhere to international regulatory protocols?
Yes, our medical devices are designed and manufactured to meet or exceed international regulatory standards, including but not limited to CE, ISO and USFDA certifications, ensuring safety and efficacy for patients worldwide.
Can I distribute your Spinal Needles in hospitals, clinics and other medical institutions?
Yes, our medical devices are designed for distribution in a variety of healthcare settings, including hospitals, clinics and other medical institutions. Our straightforward distributor verification process makes it convenient to start distributing our products. For more details on distributing our medical devices in your area, reach out to our International Sales Department.
References
Treatment and Prevention of Post-dural Puncture Headaches: A Systematic Review
This systematic review by RI Alatni, R Alsamani, A Alqefari in Cureus evaluates the effectiveness of different strategies in treating and preventing post-dural puncture headaches, a common complication associated with the use of spinal needles. It highlights the importance of needle type selection, such as pencil-point versus Quincke needles, in minimizing this risk.
Factors Predisposing to Postdural Puncture Headache after Spinal Anaesthesia among Elective Caesarean Section Patients at Thika Level 5 Hospital, Kenya
This study by MA Nduku, O Jackson, and colleagues, published in the African Journal of Medicine and Health Sciences, examines factors contributing to postdural puncture headaches in patients receiving spinal anesthesia with spinal needles for cesarean sections. It provides insights into needle selection and procedural techniques to reduce complications.

You might be interested in...
Why AdvaCare Pharma?
As an industry leader, we are aware of our responsibility to provide affordable and sustainable solutions to improve healthcare worldwide.