- Home›
- Medical Devices›
- Injection Instruments›
- Intravenous (IV) Supplies›
- Medical Guide Wire
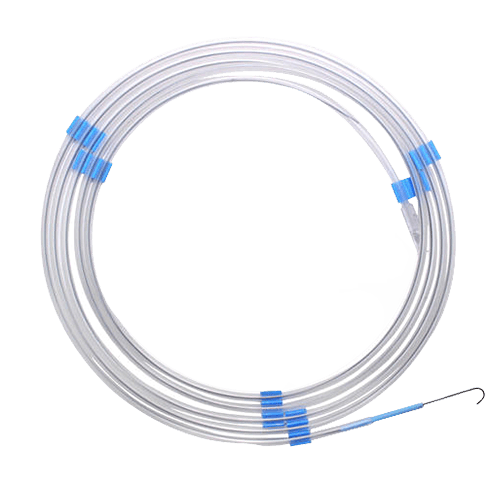
Medical Guide Wire
Material
Coating
Tip
Core Wire Type
Length
Diameter
Packaging
What is a Medical Guide Wire?
Medical Guide Wire is a thin, flexible wire used to guide the placement of medical devices such as catheters or stents during surgical or diagnostic procedures. This vascular access device is made of either stainless steel or nitinol and has either a straight or J tip. Lengths range from 145cm to 300cm.
Medical guide wires are typically inserted through a needle and advanced into the patient's blood vessels or other body cavities under imaging guidance, such as X-ray or ultrasound, to guide the placement of other medical devices. They may be used in a variety of medical procedures, including angiography, embolization, and drainage of fluid collections.
AdvaCare Pharma manufactures high quality Medical Guide Wires in China, India, and the USA. Our facilities undergo regular compliance and control assessments to ensure adherence to all health, safety, and environmental guidelines.
Product Specifications
Coating
Tip
Core Wire Type
Uncoated
Uncoated medical guide wires refer to guide wires that have not been coated with any materials that may modify their surface characteristics. Uncoated guide wires are a cost-effective option and offer a high level of tactile feedback for users, which is essential for achieving precise control when performing different types of medical procedures.
PTFE Coated
PTFE Coated medical guide wire is covered with a polymer called PolyTetraFluoroEthylene, which makes it easier to insert the device with less trauma to the patient. The coating also reduces friction during the placement of the wire, resulting in a smoother procedure. This type of guide wire is commonly used in vascular access procedures.
Hydrophilic PTFE Coated
Hydrophilic PTFE Coated medical guide wire contains has a coating that is made of PTFE and hydrophilic lubricant. This combination helps to reduce friction during insertion and navigation, particularly in wet conditions. This type of coating also helps provides a smooth ride and easy tracking, resulting in minimal trauma during the procedure.
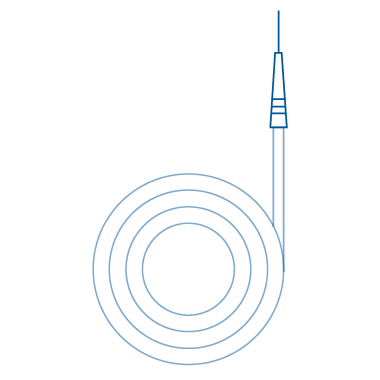
Straight
Straight medical guide wires feature a distal end that remains straight, rendering them highly suitable for procedures demanding precise navigation. These guide wires find frequent application in catheter placement, particularly in vessels with a straightforward trajectory or when the target site is easily accessible. By providing maneuverability and control, they assist healthcare professionals in achieving accurate positioning.
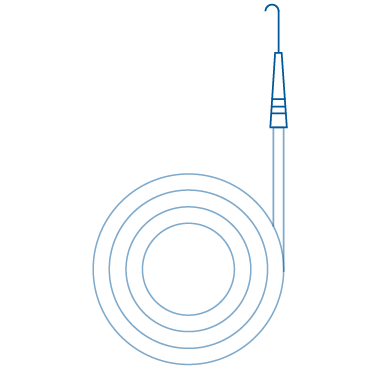
J-Tip (Angled)
J-Tip (Angled) medical guide wire has a distal end that is angled in a "J" shape to facilitate easier access to difficult-to-reach areas. This tip design can help reduce trauma to the patient's vessels during insertion and navigation, making it a valuable tool in certain procedures. This unique tip design enhances safety and precision, allowing healthcare professionals to navigate intricate anatomical structures with greater ease.
Fixed Core
Fixed Core medical guide wire is designed with a solid, non-removable core, which plays a vital role in delivering exceptional support and stability during medical procedures. This wire is particularly favored in situations that demand heightened control and utmost precision. Its reliable construction ensures optimum performance, allowing healthcare professionals to navigate with confidence and achieve successful outcomes.

Movable Core
Movable Core medical guide wire is engineered with a flexible tip that is achieved by strategically positioning the core wire away from the coating. This design finds widespread application in various endovascular procedures, including angioplasty and stenting. By offering enhanced flexibility, this guide wire enables medical professionals to navigate challenging anatomical structures with greater ease and precision.
Why are we a quality Medical Guide Wire manufacturer?
AdvaCare Pharma is a reputable manufacturer of Medical Guide Wires, which are produced in ISO and CE-certified facilities with rigorous quality control measures in place to ensure compliance with global health and safety standards.
With a presence in over 65 countries, our AccuPoint™ brand has helped numerous medical distributors succeed in their respective markets. We are committed to innovation and offer CE, ISO and FDA certifications for certain product specifications. All AccuPoint™ medical devices come with a STED dossier for product registration.
At AdvaCare Pharma, we believe in providing excellent customer service, which is why we have teams of experienced professionals who are available to provide support and answer any questions our medical distributors may have. We understand the importance of timely delivery and strive to ensure that our products reach our partners as quickly as possible through our global distribution network.
Uses
How should a Medical Guide Wire be used?
A medical guide wire should be used by a trained medical professional following the manufacturer's instructions. Before use, the packaging should be inspected for damage and the expiration date checked. The guide wire should be inserted into the device or catheter using aseptic technique, ensuring that the wire does not come into contact with non-sterile surfaces.
How should a Medical Guide Wire be disposed of?
After use, the guide wire should be disposed of in accordance with local regulations and healthcare facility policies for medical waste disposal.
FAQs
What is the difference between Medical Guide Wires made of stainless steel and nitinol?
Medical guide wires made of stainless steel are more rigid and have a higher tensile strength, while nitinol guide wires are more flexible and have shape memory capabilities. Both materials have their own advantages and may be used for different procedures depending on the requirements.
What is the difference between uncoated, PTFE coated, and hydrophilic PTFE coated guide wires?
Uncoated guide wires have a smooth surface, while PTFE coated guide wires have a non-stick surface that can reduce friction during insertion. Hydrophilic PTFE coated guide wires have a water-activated coating that provides even lower friction and improved navigation through blood vessels.
What is the difference between a J-tip and a straight-tip guide wire?
J-tip guide wires have a curved end that allows for easier access and navigation through blood vessels, while straight-tip guide wires are more rigid and can provide more precise control during insertion.
How should Medical Guide Wires be prepared before use?
Medical guide wires should be inspected for damage or defects before use, and should be sterilized according to the product instructions. It is also important to confirm the correct length and diameter of the guide wire for the intended procedure.
How should Medical Guide Wires be stored and handled?
Medical guide wires should be stored in a clean, dry place and protected from damage or contamination. It is important to handle the guide wire with care and avoid bending or kinking, as this can cause damage to the wire and affect its performance during insertion.
Which other medical instruments does your company manufacture?
We produce a comprehensive range of over 500 Class I and Class II medical devices, encompassing surgical instruments, diagnostic equipment, medical consumables, wound care products, and incontinence devices. Our products cater to hospitals, clinics, pharmacies, and healthcare facilities.

You might be interested in...
Why AdvaCare Pharma?
As an industry leader, we are aware of our responsibility to provide affordable and sustainable solutions to improve healthcare worldwide.