- Home›
- Medical Devices›
- Skin & Wound Care›
- Wound Dressings›
- IV Dressing
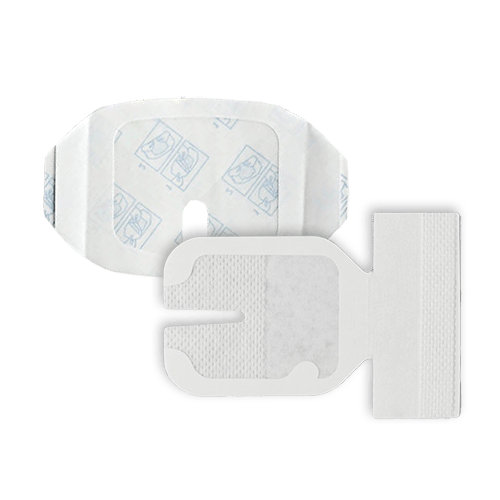
IV Dressing
Material
Shape
Size
Packaging
What is an IV Dressing?
An IV Dressing is a sterile adhesive used to secure and protect IV catheters or infusion sites and to maintain the integrity of the IV access point. It is made of either polyurethane, PE foam, or non-woven material and comes in various shapes and sizes to accommodate different medical needs.
Aside from securing IV catheters or infusion sites, IV dressings also serve to prevent infection by creating a barrier against external contaminants. They provide a sterile environment around the insertion site, reducing the risk of bacteria entering the bloodstream. The transparent nature of many IV dressings allows for easy monitoring of the insertion site, enabling healthcare professionals to observe any signs of infection or complications promptly.
Manufactured in ISO and CE-certified facilities across India, China, and the USA, AdvaCare Pharma's IV Dressings prioritize consistent quality and safety. Our manufacturing plants are regularly inspected to maintain compliance with healthcare standards.
Product Specifications
Material
Shape
Polyurethane (PU)
Polyurethane (PU) IV dressing is a reliable and resilient medical product designed to offer enhanced protection and safety. Made from durable and breathable polyurethane material, it acts as a waterproof barrier, safeguarding the insertion site from external contaminants. Its transparent nature allows healthcare professionals to easily monitor the site for any potential complications, ensuring prompt intervention when necessary.
PE Foam
PE Foam IV dressing is a lightweight, hypoallergenic, comfortable, and flexible material that helps absorb pressure, reducing the risk of skin damage or irritation around the catheter site. It also acts as a shock absorber, protecting the insertion site from external impacts.
Non-woven
Non-woven IV dressing is made of a soft, flexible, and breathable material, providing optimal comfort for patients. Its hypoallergenic nature ensures compatibility with sensitive skin, while allowing for easy application and secure fixation of the dressing. With its excellent adhesive properties, this dressing remains firmly in place even during movement, offering reliable protection for the catheter site.
Octagon
Octagon IV dressing is a versatile option designed for both PICC (Peripherally Inserted Central Catheter) and CVC (Central Venous Catheter) applications. Its hypoallergenic adhesive frame ensures compatibility with various skin types, reducing the risk of irritation. This sterile dressing is intended for single use.
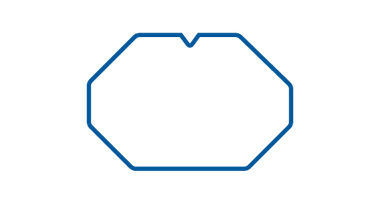
Winged
Winged IV dressing is specifically designed for infants and children, providing a secure fixation for IV cannulas. Its hypoallergenic adhesive frame ensures compatibility with sensitive skin, minimizing the risk of allergic reactions. This sterile dressing is intended for single use, offering a hygienic solution for reliable IV care in pediatric patients.
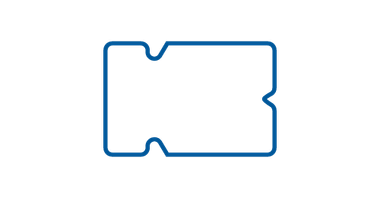
Hexagon
Hexagon IV dressing is specifically designed for the secure fixation of arterial and T-prolonged catheters. Its hypoallergenic adhesive frame ensures compatibility with various skin types, reducing the risk of irritation. This sterile dressing is intended for single use, providing a hygienic and convenient solution for arterial and T-prolonged catheter care.
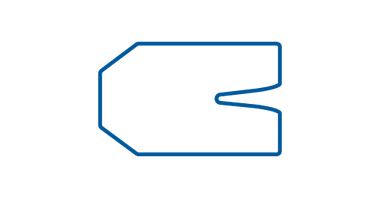
Oval
Oval IV dressing is designed to securely fixate a variety of catheters, including carotid, jugular, pulmonary, hemodialysis, and multi-lumen catheters. Its hypoallergenic adhesive frame ensures compatibility with different skin types, minimizing the risk of irritation. This sterile dressing is intended for single use, providing a hygienic and reliable solution for the care and fixation of various types of catheters.
Why are we a quality IV Dressing manufacturer?
AdvaCare Pharma is a leading global supplier in the medical supplies industry, manufacturing IV Dressings and a wide array of other wound dressings. Our dedication to excellence is reflected in our meticulously designed line of medical supplies, prioritizing maximum efficacy and safety while considering environmental impact. Our production facilities in China, India, and/or the USA strictly adhere to CE and ISO quality standards to guarantee the delivery of superior products.
For over two decades, AdvaCare Pharma established a strong presence across 65 global markets through a robust distribution network. Our success is attributed to the value we place on fostering strong partnerships with our trusted distributors and partners. By understanding their distinct requirements, we offer tailored solutions to customer satisfaction and reinforce our position as a reliable provider of top-tier medical supplies.
Uses
How should an IV Dressing be used?
Before utilizing an IV dressing, it is imperative to check that the insertion site is meticulously cleaned and thoroughly dried to minimize the risk of infection:
- Carefully remove the backing from the adhesive side of the dressing, taking the utmost care to avoid any contact with the adhesive surface.
- Gently apply the dressing over the catheter insertion site, verifying complete coverage and a secure seal to prevent any ingress of contaminants.
- Smooth down the edges of the dressing meticulously to guarantee ideal adhesion and to mitigate the likelihood of any lifting or displacement, which could compromise the dressing's efficacy in protecting the IV access point.
- Consider any specific instructions provided by healthcare professionals regarding the application technique, as adherence to dressing protocol upholds the integrity of the IV site and minimizes the risk of complications.
How should an IV Dressing be disposed of?
When it comes to the disposal of an IV dressing, it is mandatory to handle it with caution and adhere to proper medical waste disposal protocols to preserve hygiene and minimize any potential risks of contamination.
To dispose of the IV dressing, begin by carefully peeling it off from one side, while simultaneously holding the surrounding skin taut with the other hand to minimize any discomfort or irritation. Once removed, promptly place the used dressing in a designated biohazard or medical waste container, checking that it is securely sealed to prevent any spillage or exposure to pathogens.
Remember to label the container appropriately to indicate its contents and follow the prescribed guidelines for disposal. Adequate disposal of medical waste is required not only for maintaining a safe and hygienic healthcare environment but also for protecting public health and the environment at large.
What are the main considerations when selecting an IV Dressing for clinical use?
When selecting an IV dressing for clinical use, several factors are to be taken into account to allow for best patient outcomes and safety:
- The type of catheter or infusion device being utilized should dictate the choice of dressing shape and size for the best coverage and secure fixation.
- Consideration should be given to the patient's skin condition and sensitivity, as some materials may cause irritation or allergic reactions. Transparency and breathability are main features to encourage ongoing monitoring of the insertion site and nurture skin health.
- The dressing's adhesive properties and ability to maintain secure adhesion, even in high-mobility areas, are major considerations to prevent dislodgement and minimize the risk of complications.
- Selecting the most appropriate IV dressing involves a careful assessment of patient needs, device characteristics, and environmental factors to guarantee efficient and safe catheter management.
What part does applying dressings correctly have in preventing IV-related complications?
Good dressing application is paramount in preventing IV-related complications and preserving the integrity of the IV access point. A correctly applied dressing serves as a protective barrier against microbial contamination, reducing the risk of bloodstream infections and other complications.
Through creating a sterile environment around the insertion site, the dressing aids in minimizing the ingress of pathogens and foreign particles, which could lead to infection or inflammation. Secure fixation of the catheter with an appropriately applied dressing prevents accidental dislodgement, which can disrupt therapy delivery and increase the risk of catheter-related bloodstream infections (CRBSIs).
FAQs
How does an IV Dressing work?
An IV dressing works by providing a protective barrier over the insertion site of an IV catheter or infusion site. It helps to secure the catheter in place and maintain its integrity, reducing the risk of infection and ensuring the delivery of fluids or medications.
What are the differences between PU, PE foam, and non-woven IV Dressings?
PU (polyurethane) IV dressings are transparent and flexible, allowing for easy monitoring of the insertion site. PE foam dressings are soft and absorbent, providing cushioning and protection. Non-woven dressings are breathable and hypoallergenic, suitable for sensitive skin. The choice of material depends on factors such as transparency needs, moisture management, and patient skin sensitivity.
What are the differences between octagon, winged, hexagon, and oval-shaped IV Dressings?
Octagon-shaped IV dressings are designed to conform to different body contours, providing a secure fit. Winged IV dressings have additional adhesive wings that help with anchoring and stabilization. Hexagon-shaped dressings offer a larger surface area for better adhesion, especially in high-mobility areas. Oval-shaped dressings are versatile and can be used in various locations, including joints. The selection of shape depends on the specific needs of the patient and the insertion site.
Can I order customized configurations of your medical devices?
Yes, we offer customization options for many of our IV dressings to accommodate specific user preferences and clinical requirements. We employ agile production methods to increase our manufacturing capability specifically to offer a wider range of specifications that can meet the needs of every market.
How do your IV Dressings compare to traditional dressings on the market?
Unlike traditional dressings, our medical IV dressings are specifically designed to address the unique challenges associated with IV catheter management. With their advanced adhesive formulation and innovative design features, our dressings offer enhanced stability, durability, and patient comfort. Their transparent construction allows for continuous visualization of the IV site, minimizing the need for dressing changes and reducing the risk of catheter dislodgement or infection.
How should IV Dressings be stored and handled?
IV dressings should be stored in a cool, dry place, away from direct sunlight and extreme temperatures. Maintain the sterility of the dressings by handling them with clean hands or wearing gloves. Follow the manufacturer's instructions regarding proper storage and expiration dates to ensure the dressings remain effective and safe for use.
References
Peripheral Intravenous Catheter Dressing and Securement Practice
This study compared different dressing and securement practices for peripheral intravenous catheters. It found that sterile gauze and tape dressings were associated with fewer insertion site complications and better dressing integrity compared to non-bordered polyurethane dressings. Moreover, non-sterile tape at the insertion site was linked to more complications. The research highlighted the importance of proper dressing and securement techniques in reducing catheter-related complications.

You might be interested in...
Why AdvaCare Pharma?
As an industry leader, we are aware of our responsibility to provide affordable and sustainable solutions to improve healthcare worldwide.