- Home›
- Medical Devices›
- Injection Instruments›
- Enteral Feeding Sets›
- EVA Infusion Bag
EVA Infusion Bag
Components
Material
Volume
Packaging
What is an EVA Infusion Bag?
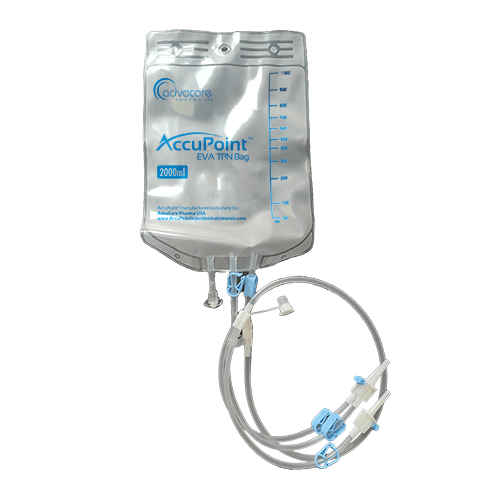
EVA Infusion Bag is a flexible, medical-grade plastic bag used to store and administer fluids or medications intravenously. It comes in volume capacities ranging from 100ml to 5000ml. EVA bags are used for various medical treatments such as hydration, nutrition, chemotherapy, and blood transfusions.
EVA infusion bags are preferred over glass bottles as they are lighter, more durable, and less prone to breakage, reducing the risk of injury and contamination. The bags are also easier to store, transport, and dispose of, making them a cost-effective and convenient solution for medical facilities.
AdvaCare Pharma is a global manufacturer of high-quality EVA Infusion Bags. Our facilities located in China, India, and the USA are regularly assessed to ensure that they comply with the most stringent health, safety, and environmental regulations.
Product Specifications
Components
EVA Infusion Bag consists of a 3-way valve, plastic dropper, tube, roller clamp, and adapter with cap. It features a luer lock connector for easy filling and infusing and an ergonomic spike for secure connection. This bag is suitable for usage with both gravity and pump enteral feeding sets.
Material
EVA Infusion Bag is crafted from Ethylene Vinyl Acetate (EVA), a non-PVC material that offers several advantageous features. It is DEHP and Latex-free, making it safe and suitable for a wide range of patients. EVA's flexibility, durability, and chemical resistance make it an ideal choice for reliable and secure infusion solutions.
Why are we a quality EVA Infusion Bag manufacturer?
AdvaCare Pharma is a leading manufacturer of EVA Infusion Bags, available under the AccuPoint™ brand, and produced in ISO and CE-certified facilities. Our medical devices undergo rigorous quality control measures, including testing and inspections, to ensure compliance with global health, safety, and environmental standards. With a strong presence in over 65 countries, our high-quality medical devices have helped numerous partners succeed in their respective markets.
To ensure that our partners receive only the best-quality products, AdvaCare Pharma employs a meticulous approach in every step of our production process. Our teams are committed to innovation, and we offer CE, ISO and FDA certifications for certain product specifications. Additionally, all AccuPoint™ medical devices are accompanied by a STED dossier.
We are dedicated to partnering with our medical distributors to help them achieve success in their respective markets, and we are always striving to improve our products and processes to better serve our customers.
Uses
How are EVA Infusion Bags used in hospitals?
In hospital environments, EVA Infusion Bags aid in administering fluids or medications directly into the bloodstream:
- The initial step involves a detailed evaluation of the patient's needs to determine the suitability of IV therapy.
- Sterility is a top priority; healthcare workers meticulously clean their hands prior to handling the infusion setup.
- Positioned appropriately on IV stands, these bags deliver a steady flow into the patient's vein through sterile equipment, which includes a roller clamp for flow rate adjustment, and various connectors for a secure setup.
- The insertion site on the patient is prepared with careful cleaning, followed by the insertion of a sterile IV catheter by a nurse or a doctor.
- Monitoring the infusion's progress is continuous to safeguard the therapy's success and patient safety.
- Post-treatment, the bag's disposal complies with strict protocols, emphasizing environmental safety and infection control.
How are EVA Infusion Bags used in clinics and medical centers?
Clinics and medical centers employ EVA Infusion Bags in procedures mirroring those in hospitals:
- Practitioners assess each case to decide on IV therapy's necessity, tailoring the fluid or medication type and volume to the patient's specific condition.
- The selected EVA Bag is prepped for use, with staff checking it is free from damage and correctly connected to the IV line.
- The patient's comfort and the precise delivery rate of the therapy are carefully managed throughout the procedure, with adjustments made as required based on ongoing monitoring.
- Following the session, the used bag is disposed of in compliance with health and safety guidelines, maintaining a focus on sterility and patient well-being.
How do doctors and practitioners use EVA Infusion Bags in patient care?
EVA Infusion Bags are integral to patient care, offering a method to administer much needed treatments. Doctors determine the need for such therapy, specifying the solution and rate to meet the patient's health requirements.
Practitioners prepare the bag, confirming sterility, and connect it to the patient via an IV line, with the flow controlled to match the treatment plan. This process demands constant vigilance to adjust as needed and to observe the patient's reaction to the infusion.
After completing the treatment, healthcare providers follow strict procedures for bag disposal, establishing a clean and safe environment.
How should EVA Infusion Bags be used safely in clinical practice?
Safe application of EVA Infusion Bags in clinical settings hinges on meticulous preparation and adherence to procedural standards:
- Selection of the appropriate bag type and volume is the first step, followed by strict hand cleanliness to avoid contamination.
- Each bag must be inspected for integrity before use. Employing a sterile method for catheter introduction minimizes infection risks.
- Throughout the treatment, careful monitoring of the infusion rate and patient condition is of the utmost importance.
- After use, the disposal of the EVA Bag must comply with health and safety regulations, emphasizing the importance of environmental and patient safety protocols.
How are EVA Infusion Bags used for enteral nutrition?
For enteral nutrition, EVA Infusion Bags are instrumental in delivering nutritional solutions directly to the gastrointestinal tract via feeding tubes.
These bags, filled with nutrient-rich solutions, connect to tubes leading to the patient's stomach or intestines, by passing the need for oral intake.
This method is particularly beneficial for patients unable to consume food normally due to various health conditions, allowing them to receive adequate nutrition for recovery and health maintenance.
How do EVA Infusion Bags contribute to patient comfort and safety?
EVA Infusion Bags strengthen the healthcare experience by providing a safer, more comfortable alternative to rigid containers.
Their lightweight nature, combined with the absence of breakage risks, assists in easier handling and reduces injury risks. The material's flexibility and compatibility with various infusion setups provides aid to a smooth, controlled delivery of therapies.
Being DEHP and latex-free broadens their suitability across patient groups, including those with specific sensitivities. The design elements of these bags, such as secure connectors and valves, further prevent leakages and disconnections, underlining their role in promoting safety and comfort in medical treatments.
FAQs
What is EVA, the material used to make infusion bags?
EVA stands for ethylene-vinyl acetate, a flexible, medical-grade plastic that is biocompatible and safe for storing and administering fluids and medications intravenously.
What are the different volume capacities of EVA Infusion Bags, and where are they typically used?
EVA infusion bags come in volume capacities ranging from 100ml to 5000ml and are used in various medical treatments such as hydration, nutrition, chemotherapy, and blood transfusions. Smaller bags may be used for pediatric patients, while larger bags are suitable for adult patients or for longer-term treatments.
How should EVA Infusion Bags be stored and handled?
EVA infusion bags should be stored in a clean, dry place at room temperature, away from direct sunlight and heat sources. Before use, the bag should be visually inspected for any signs of damage or leaks. Care should be taken to ensure that the bag is not punctured or damaged during handling or transport.
What are the different parts of an EVA Infusion Bag, and how do they work?
An EVA infusion bag typically includes a 3-way valve, dropper, tube, roller clamp, and adapter with cap. These parts work together to allow for the safe and controlled administration of fluids or medications intravenously. The 3-way valve controls the flow of the fluid, while the roller clamp allows for easy adjustment of the flow rate.
How should EVA Infusion Bags be disposed of after use?
EVA infusion bags should be disposed of in accordance with local regulations and healthcare facility policies. Empty bags can be placed in regular waste bins, while bags that have contained hazardous or infectious materials should be disposed of in accordance with relevant guidelines. Care should be taken to ensure that the bag is completely empty before disposal.
Are your EVA Infusion Bags customizable to meet specific market needs?
Yes, we offer customization options for many of our medical devices to accommodate the specific needs and preferences of different markets and patient populations. However, with 1M+ specification options available, our standardized product specifications usually meet the needs of every market.
Can I request samples of your EVA Infusion Bags for evaluation?
We do provide samples of our medical devices to aid in evaluation and product registration. Contact our International Sales Department to request samples tailored to your specific requirements.
Can I receive assistance with regulatory matters for importing your EVA Infusion Bags?
Yes, we extend regulatory support and guidance to our distributors, aiding in adherence to local regulations and facilitating the smooth importation of our medical devices. Backed by our proficient Regulatory Affairs Department, comprising experts such as pharmacists, biomedical engineers, QA professionals, and documentation specialists, we ensure a seamless product registration process.
References
Physical characteristics of total parenteral nutrition bags significantly affect the stability of vitamins C and B1
This study investigated the stability of vitamins in total parenteral nutrition (TPN) mixtures stored in EVA bags. It was found that the stability of vitamins, especially vitamin C, was significantly affected by the type of bag used. Vitamin C was more stable in multilayered bags compared to EVA bags, with considerable degradation occurring in EVA bags at various temperatures. This study is crucial for understanding how the material of infusion bags can influence the stability of nutrients in TPN solutions.
Vitamin stability in a TPN mixture stored in an EVA plastic bag
This research focused on the stability of various vitamins in a TPN admixture stored in EVA bags. It highlighted that while most vitamins remained stable, ascorbic acid and folic acid showed significant degradation. This study sheds light on the importance of considering the type of storage bag for maintaining the efficacy of vitamin-containing parenteral nutrition solutions.

You might be interested in...
Why AdvaCare Pharma?
As an industry leader, we are aware of our responsibility to provide affordable and sustainable solutions to improve healthcare worldwide.