- Home›
- Medical Devices›
- Diagnostic Detection Devices›
- Rapid Test Kits (RDT)›
- Dengue Test Kits
Dengue Test Kits
Type
Specimen
Form
Packaging
What are Dengue Test Kits?
Dengue Test Kits are medical diagnostic tools that are used to detect antigens or antibodies due to infection with the virus that causes dengue fever. Dengue fever is a viral illness that is commonly transmitted by the Aedes mosquito, and this disease is prevalent in many regions of the world.
Dengue test kits are produced in three variations: dengue IgG/IgM antibody test kits, dengue NS1 antigen test kits, or IgG/IgM/NS1 combo test kits. Each of these test kits is intended for the qualitative detection of dengue virus-specific IgG and IgM antibodies and NS1 antigens in the case of acute dengue virus infection.
Dengue fever test kits are recommended for professional use only and are not intended for at-home use.
The Dengue Test Kits are manufactured by AdvaCare Pharma, a leading pharmaceutical company. These infectious disease test kits have been produced in strategically-located facilities across India, China, and the USA.
Product Specifications
Type
Specimen
Form
Dengue IgG/IgM Antibody Test Kit
Dengue IgG/IgM Antibody Test Kit is used to detect the presence of IgG and IgM antibodies specific to the dengue virus in whole blood or a serum/plasma sample.
The test is conducted using a cassette format, which allows for easy and quick interpretation of results. It is an effective tool for the early diagnosis of dengue fever and can assist healthcare professionals in determining the immune response to the infection.
Dengue NS1 Antigen Test Kit
Dengue NS1 Antigen Test Kit is a diagnostic tool used to detect the presence of the NS1 antigen of the dengue virus in whole blood or serum/plasma samples.
The test kit comes in a cassette format, which allows for easy and accurate interpretation of results. It is a valuable tool for early diagnosis of dengue infection, as the NS1 antigen can be detected during the early stages of the disease.
Dengue IgG/IgM/NS1 Combo Test Kit
Dengue IgG/IgM/NS1 Combo Test Kit is a comprehensive diagnostic tool used to detect multiple markers associated with dengue infection.
The combo test kit is produced as a multi-panel cassette format, which allows for the simultaneous detection of dengue IgG antibodies, IgM antibodies, and NS1 antigens in whole blood or serum/plasma samples.
Whole Blood
Whole Blood sample required may be obtained using standard venipuncture (2 drops) or fingerstick (50 μL) techniques. For serum or plasma samples, the specimen must be centrifuged. The sample is then transferred to the designated wells on the cassette of the cassette. A drop of the corresponding buffer dilution must be added afterward to initiate the test.
Serum/Plasma
A Serum/Plasma specimen is needed for this type of test. Once the blood samples have been centrifuged to separate the serum/plasma, the resulting specimen needs to be carefully transferred to the designated areas of the cassette using a capillary tube or dropper.
Following this, a single drop of the buffer dilution should be promptly added to the specimen. The buffer and sample will then interact, which will lead to a test result.
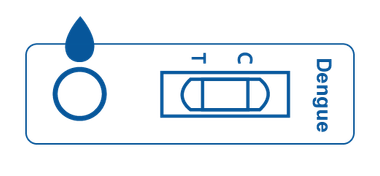
Cassette
Cassette is a plastic container with a test strip inside. The plastic box features a designated area for sample application and an area for displaying positive, negative, or invalid test results.
The test strip inside of the cassette is designed to react with the designated antibody or antigen.
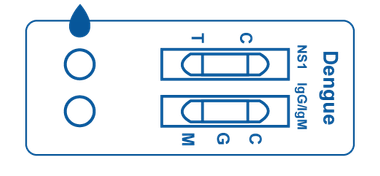
Multi-Panel Cassette
Multi-Panel Cassette consists of a plastic box that contains 2 test strips inside. Each cassette includes two designated areas for sample placement and two windows that display lines, which represent positive, negative, or invalid test results.
The test strips inside contain reagents which interact with any present antibodies or antigens in the patient sample.
Why are we a leading Dengue Test Kits manufacturer?
AdvaCare Pharma is a leading manufacturer of Dengue Test Kits, a medical test in the AccuQuik™ brand of diagnostic detection devices. Our production facilities are held to stringent CE and ISO standards. As a manufacturer of rapid test kits, AdvaCare Pharma partners with an extensive global network of institutions, including hospitals, pharmacies, distributors, and more.
For 20 years, AdvaCare Pharma has been dedicated to providing premium and affordable pharmaceutical products for an ever-changing global community. We prioritize our partners and pride ourselves on building lasting and mutually beneficial relationships.
Uses
How should Dengue Test Kits be used?
To use AccuQuik™ Dengue Test Kits, follow these simplified steps:
- Sample Collection: Collect the patient's whole blood, serum, or plasma sample. Utilize standard venipuncture techniques for blood collection or a fingerstick method for whole blood samples.
- Administering the Specimen: Apply the collected sample to the designated sample well on the cassette accurately, as per the provided instructions with our AccuQuik™ Dengue Test Kits.
- Adding the Buffer: Immediately add the buffer solution to the cassette following the specimen application. The buffer is central for the test's reaction and the accuracy of results.
- Waiting for Results: Let the cassette sit undisturbed for 15 minutes to process the sample. The kits are engineered to deliver results within this timeframe for best accuracy. Do not to interpret the results after 20 minutes as this could affect the test's reliability.
- Interpreting Results: Results are ready to be interpreted at 15 minutes. The test cassette will display lines to indicate whether the test is positive, negative, or invalid.
How should Dengue Test Kits be disposed of?
Correct disposal of AccuQuik™ Dengue Test Kits is mandatory to prevent any potential health hazards. After conducting the test, all components of the kit should be considered as biohazardous material. These components, including the used cassette, gloves, and any other items that came into contact with the sample, should be placed in a biohazard bag.
This bag must be sealed securely and disposed of in an appropriate biohazard waste container to comply with local health and safety regulations. This careful approach to disposal helps safeguard healthcare workers, patients, and the broader community from potential exposure to infectious agents.
What are the storage specifications for Dengue Test Kits?
AccuQuik™ Dengue Test Kits must be stored properly to maintain their efficacy and reliability. The recommended storage condition is a temperature range of 2-30°C (36-86°F), in a location that is shielded from direct exposure to sunlight and sources of heat or moisture. These conditions help preserve the reactive elements within the kits, assuring they perform accurately when deployed.
Keep the kits in their original packaging until they are ready to be used, further protecting them from environmental factors and potential contamination. This meticulous approach to storage is all-important for sustaining the kits' integrity over time and guaranteeing that they provide dependable results whenever they are needed.
Are there any factors that could affect the accuracy of Dengue Test Kit results?
While AccuQuik™ Dengue Test Kits are designed for high reliability, several external factors can potentially affect the accuracy of the results. These factors include:
- Improper sample collection, such as insufficient sample volume or contamination.
- Failure to adhere to the specified procedural steps.
- Inadequate storage conditions that expose kits to extreme temperatures or moisture.
- Technical errors during the test administration.
- The presence of similar viruses in the sample may cause cross-reactivity, leading to false positives or negatives.
FAQs
How does a Dengue Test Kit work?
Within the cassette device, each test strip contains a dengue virus-specific antigen or antibody that will bind to its designated antibody or antigen. This will result in the appearance of lines on the test strip. False positives or false negatives may occur due to factors such as technical or procedural errors or inadequate specimen collection or storage.
Which type of Dengue Test Kits should be performed?
The recommended test kit type is dependent on the clinical situation and the stage of the illness of the patient. Dengue IgG/IgM antibody test kit is able to detect the presence of virus-specific IgG and IgM antibodies within a sample. Typically, IgM antibodies are detectable during acute infection, and IgG antibodies are detectable for months or years after infection. This type of dengue fever test is recommended for evaluating a past or recent dengue virus infection. It is not usually able to detect the virus early in the infection.
The dengue NS1 antigen test kit is able to detect the virus antigen NS1 within the acute stage of infection. It is not able to detect a past infection.
The dengue IgG/IgM/NS1 combo test kit is a more comprehensive method of testing, which can provide a clearer indication of the stage of infection. It is a recommended method for settings with limited lab resources.
Who should be tested with Dengue Test Kits?
The World Health Organization (WHO) recommends diagnostic testing for patients with fever and presenting symptoms of a dengue virus infection, particularly in regions in which dengue is endemic or epidemic or who have recently traveled to an area with a high dengue transmission rate. It is also recommended to test patients exhibiting symptoms who may have been exposed to the virus through blood transfusion or organ transplant.
What are the limitations of using a rapid Dengue Test Kit?
Though the dengue fever testing kits are reliable, there are known cross-reactivity with closely-related viruses, such as the zika virus. These tests should be used in conjunction with other diagnostic tools for clinical evaluation.
Is it possible for me to distribute your Dengue Test Kits in my region?
Yes, we welcome partnerships with distributors in foreign countries who are interested in distributing our Class I and Class II medical devices within their markets. Contact our International Sales Department for more information and details about distribution in your country.
Are your Dengue Test Kits suitable for sale to medical institutions?
Yes, our medical devices are suitable for distribution in various healthcare settings, including labs, hospitals, clinics, pharmacies, and other medical institutions. With our streamlined distributor verification process, getting started is simple. Reach out to our International Sales Department to learn more about distributing our medical devices in your market.
Are your Dengue Test Kits compliant with international regulatory standards?
Yes, our medical devices strictly adhere to international regulations, encompassing rigorous certifications like CE, ISO, and/or USFDA, to ensure utmost safety and efficacy on a global scale.
References
CDC Guidance on Dengue Testing
The Centers for Disease Control and Prevention (CDC) offers detailed guidance on testing for dengue virus infection. It emphasizes that patients with symptoms consistent with dengue fever should be tested using appropriate diagnostic tests, including NS1 antigen tests, IgM antibody tests, and, when necessary, molecular tests for viral detection. The CDC underscores the importance of utilizing these tests based on the patient's symptoms and the time since symptom onset, highlighting the critical role of serologic and antigen-based tests for early and accurate diagnosis.

You might be interested in...
Why AdvaCare Pharma?
As an industry leader, we are aware of our responsibility to provide affordable and sustainable solutions to improve healthcare worldwide.















